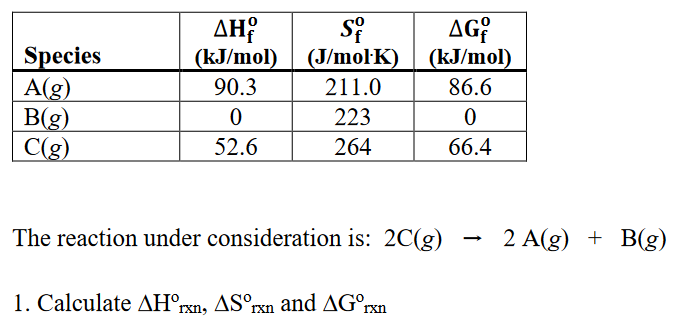
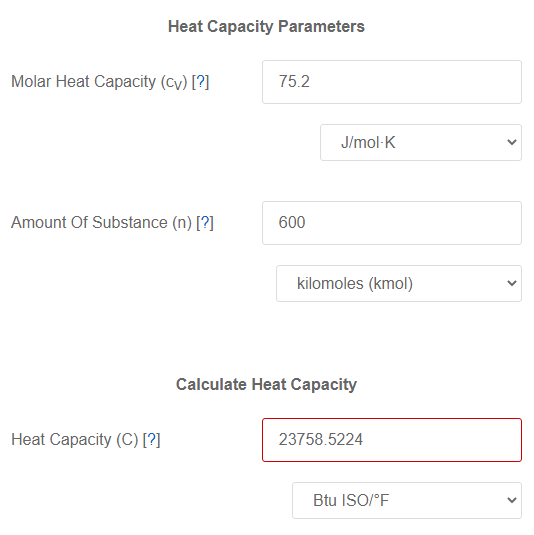
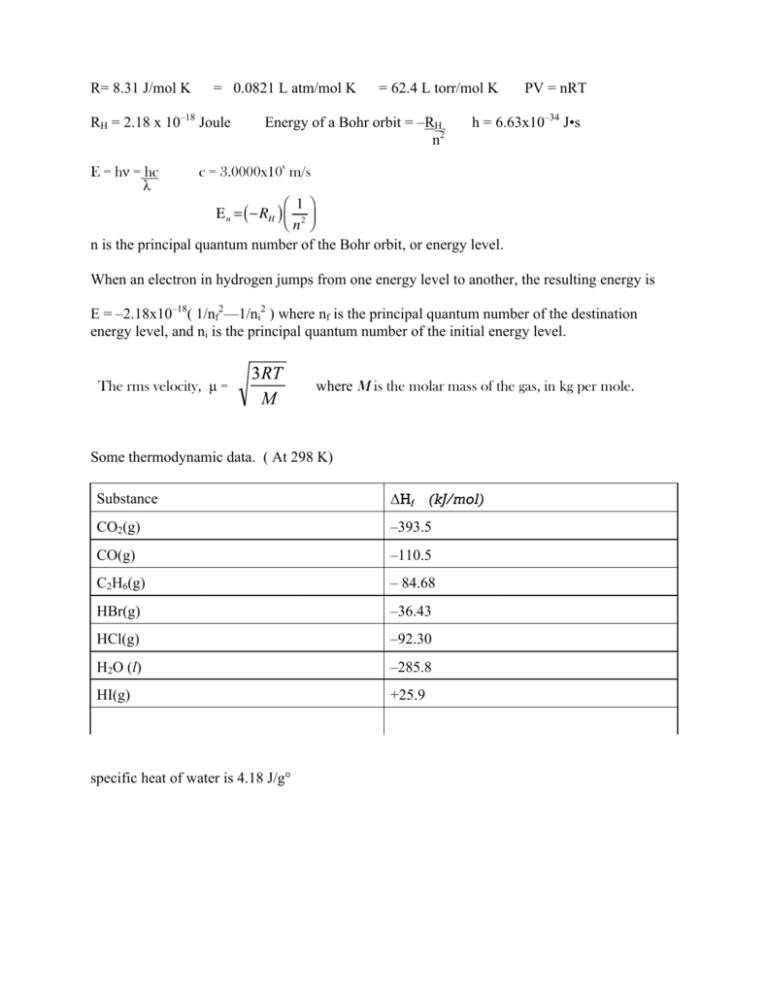
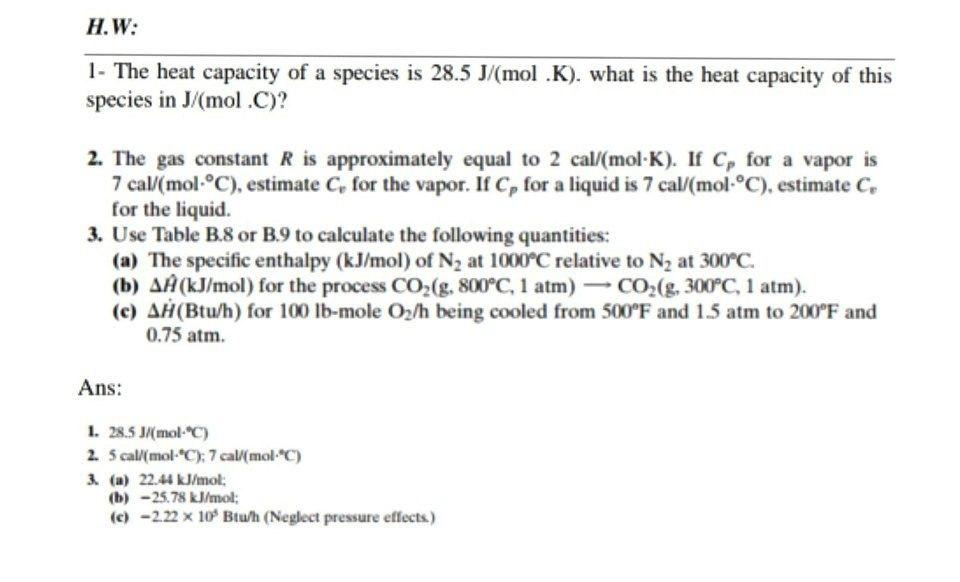
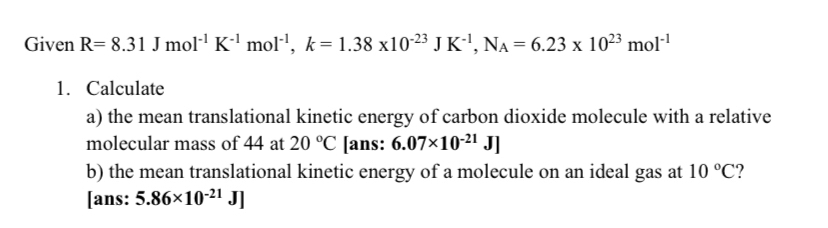Three moles of an ideal gas are taken around the cycle abc shown in the figure. For this gas, C_ p = 29.1\ \frac{J}{mol.K}. Process ac is at constant pressure, process ba
36.) Calculate the rms speed of an ideal diatomic gas having molecular weight 32 gm/mol at Oc If the specific heats at constant pressure and volume are respectively 29.1 J mol1 K
58.03 kj/ mole at 0C Cpof liquid water = 75.3j/mol/k Cpof solidvwater = 36.8 jmol//Calculate the enthalpy on freezing of 1 mole of water at 10*C to ice at 10*C enthalpy of fus. 6.
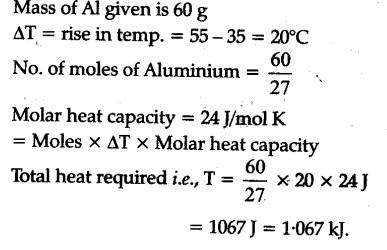


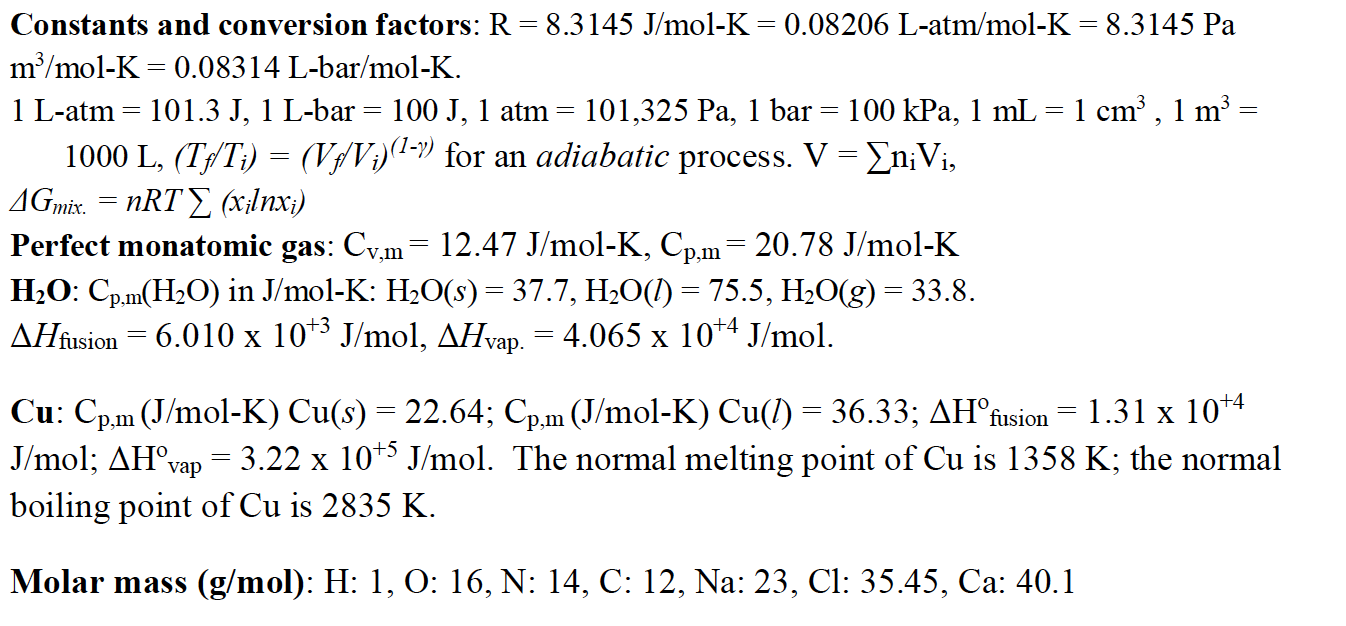


![ANSWERED] What is the internal energy of 7.00 mol o... - Physical Chemistry - Kunduz ANSWERED] What is the internal energy of 7.00 mol o... - Physical Chemistry - Kunduz](https://media.kunduz.com/media/sug-question/raw/52923887-1659262298.2603226.jpeg)